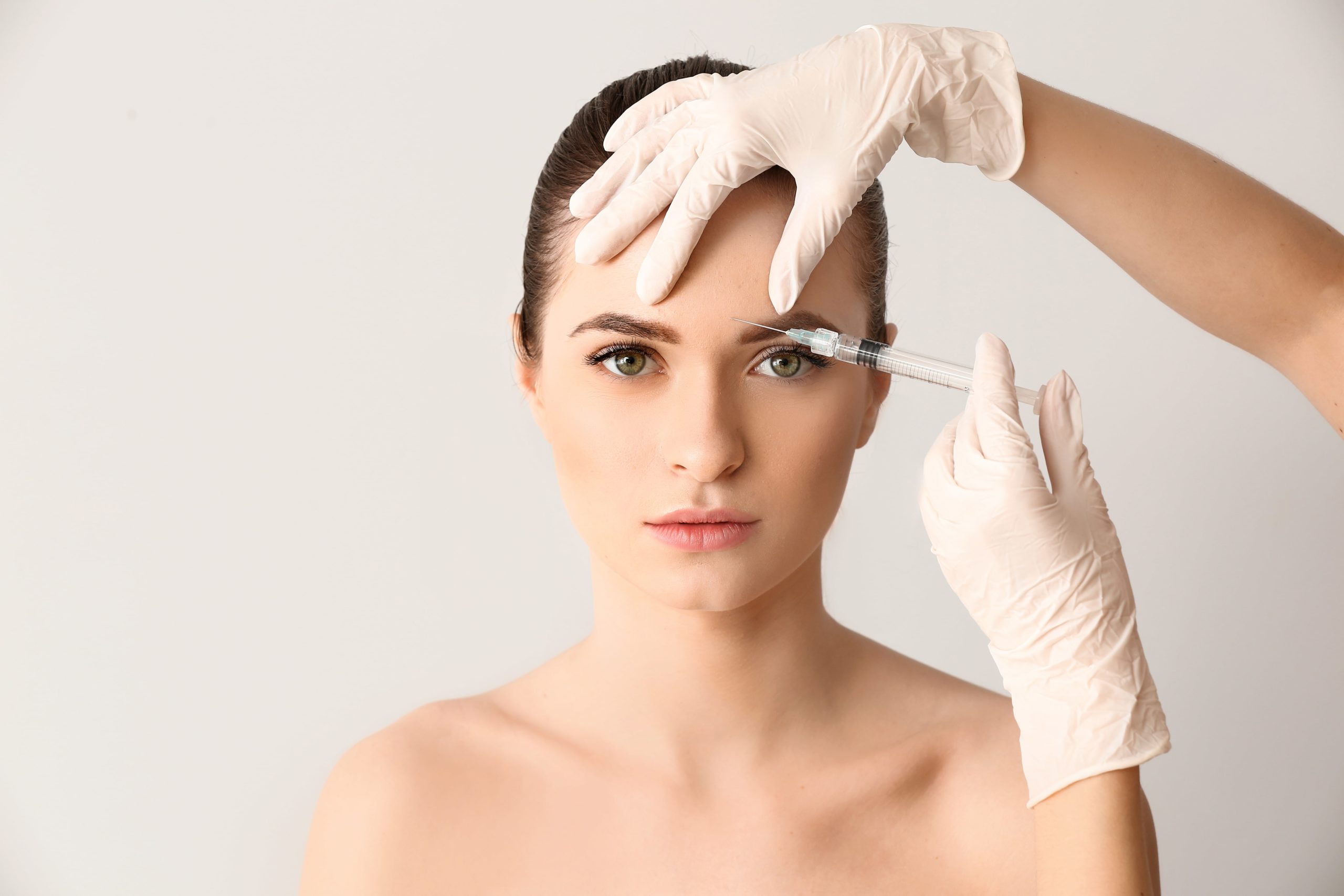
Daxxify, also known as daxibotulinumtoxina-lanm or Daxi, was created by the Nashville-based company Revance Therapeutics Inc (Nasdaq: RVNC) and is a part of a family of neuromodulators that use injections of a small amount of botulinum toxin to smooth wrinkles for a more youthful appearance. Similarly to other neuromodulators, such as Botox, Xeomin, Dysport, and Jeuveau, Daxxify is used to treat dynamic wrinkles, meaning wrinkles that develop due to repeated muscle movement. Daxxify has received FDA approval specifically to treat the glabellar lines, also known as frown lines, that appear as vertical lines on the forehead extending upward between the eyebrows. Severe frown lines can be one of the tell-tale signs of aging, and Daxxify works to relax the muscles responsible for the lines to create a natural, youthful look. Daxxify differs from other neuromodulators on the market in that it is particularly long-lasting. One of the reasons for the longevity of the effects of Daxxify is its peptide makeup. Daxxify is the first botulinum toxin injectable treatment made up of a synthetic, proprietary Peptide Exchange Technology, meaning it does not have any human or animal products. Revance’s chief executive officer, Mark Foley, says that it was the discovery of this technology that helped to stabilize Daxxify and contributed to the unique, long-lasting results.
Thank you for reading this post, don't forget to subscribe!How does Daxxify work to treat wrinkles?
Daxxify is a neuromodulator that works to smooth dynamic wrinkles. Dynamic wrinkles form as a result of repeated muscle contraction in a given area. For example, facial expressions can result in dynamic wrinkles, including smiling, which can lead to smile lines around the mouth and crows feet on the edges of the eye, and frowning or furrowing the brow, which can lead to horizontal forehead lines or frown lines in between the eyebrows. When we are young, dynamic wrinkles only appear while we are making those facial expressions and then the lines smooth when the face is at rest. However, as we age, dynamic wrinkles become etched into the skin, forming wrinkles even when the face is at rest. These tell-tale signs of the natural aging process can be reversed with treatments available on the aesthetic market, such as Botox and now Daxxify.
Daxxify works via the injection of the neuromodulator daxibotulinumtoxina-lanm. When injected into the muscle responsible for dynamic wrinkles, this neuromodulator works to block the nerve signal that tells the muscle to contract. As a result, the muscle is no longer engaged, smoothing out wrinkles in the area and preventing new wrinkles from forming. This temporary anti-wrinkle improvement of the Daxxify treatment can be observed for up to six months, making it the longest-lasting injectable neuromodulator available on the aesthetic market.
When was Daxxify FDA approved?
Daxxify was approved by the U.S. Food and Drug Administration (FDA) on September 8th, 2022. The product was set to be approved by the FDA back in 2020, but the Covid-19 pandemic delayed the FDA representative’s visit to the Revance factory for inspection. Once the factory was properly inspected, and Revance made the necessary changes to its operation, the product was finally approved in the United States. Part of receiving FDA approval for a product is to have the treatment go through rigorous clinical testing to determine its safety and effectiveness. In clinical trials, Daxxify was shown to be 98% effective after one month of treatment, with 80% of patients still noticing effects after 4 months and roughly half experiencing improvements still after 6 months. Some patients were still seeing the results of the treatment after nine months. In clinical trials, no serious side effects were observed as a result of the treatment.
Currently, Daxxify is only FDA approved to treat the frown lines, or vertical lines that appear between the eyebrows when you frown or furrow your brow. However, Revance is seeking approval for Daxxify treatment on other facial wrinkles, such as forehead lines and crow’s feet. Furthermore, Revance believes that, like Botox injections, Daxxify can be used to treat medical conditions such as neck spasms, excessive sweating, and cervical dystonia.
Daxxify vs. Botox: What are the similarities and differences between Botox and Daxxify?
There are many similarities between Botox injections and Daxxify injections. Both are neuromodulators that work in much the same way by blocking the nerve signals from the brain to the muscle, resulting in the cessation of muscle contractions to smooth out dynamic wrinkles. Both Botox and Daxxify are FDA approved to treat glabellar lines. However, they differ in that Botox is also approved to treat other cosmetic concerns, such as forehead lines and crow’s feet, and medical concerns, such as upper limb spasticity, cervical dystonia, excessive sweating, and overactive bladder. Daxxify is expected to be considered for off-label treatment of these conditions – meaning it will work to treat these conditions but has not been FDA approved to treat them. While Botox and Daxxify work in similar ways to smooth out wrinkles, they differ in their composition in that Daxxify is the only neuromodulator available on the market that does not use human or animal products as part of the composition. Botox is made from what is called human serum albumin, whereas Daxxify uses a proprietary Peptide Exchange Technology. Revance representatives believe that this contributes to Daxxify’s longevity. Botox and Daxxify also differ in how long the effects of the treatment last, with Botox results lasting for 3-4 months and Daxxify results lasting for up to 6 months.
Does Daxxify have any side effects?
Clinical trials demonstrated that Daxxify does not have significant side effects or safety concerns. As with any injectable treatment, patients may experience redness, swelling, bruising, or mild discomfort at the injection site for up to seven days after treatment. These mild side effects will resolve themselves on their own. Dr. Green recommends that patients avoid taking blood thinners before treatment in order to minimize the chance of bruising at the injection site. Clinical trials did reveal some uncommon side effects of Daxxify treatment, including headache (6% of patients) and eyelid ptosis or drooping eyelid (2%) of patients.
Why is Daxxify trending?
September 8th, 2022, the day of Daxxify’s FDA approval, saw many important headlines, including the passing of Queen Elizabeth II and the succession of King Charles. On that day, however, Daxxify was trending as well due to FDA approval of the innovative new treatment. Daxxify is poised to become an important player in the facial injectables market and could become a major competitor for the market leader, Botox. FDA approval for the treatment has been in the works for years, and with the official approval released, patients can expect the Daxxify treatment to become available sometime in 2023.